It hasn’t been a great year for parents with newborn babies. The massive Abbott baby formula recall in early 2022 triggered a nationwide product shortage. We then saw additional recalls and baby formula warnings. Mother’s Touch baby formula is the latest product to face a public alert and a recall action.
The US Food and Drug Administration (FDA) issued an alert, warning parents not to feed the product to infants even if it’s still available for sale. The agency explains that Mother’s Touch sold the product as infant formula without the pre-market notification to the FDA.
That means it wasn’t tested and facilities haven’t been inspected. Moreover, the product does not meet infant formula nutrient requirements for seven nutrients.
Mother’s Touch baby formula warning and recall
The FDA issued a warning about the issues with Mother’s Touch baby formula. Recently, the FDA updated the public alert with new information.
The agency says Mother’s Touch initiated a voluntary recall of its product last week. The FDA says that Mother’s Touch provided the information in the initial FDA warning to the affected retail consignees where the product was available for purchase. The recall notice will also be posted in prominent locations in the following areas where the baby formula was available:
- Scenic Ridge Foods in Loganton, PA 17747
- Hillside Bulk Foods in Gap, PA 17527
- Creekside Foods in Kinzers, PA 17535
The FDA says in its warning update that Mother’s Touch advises consumers who have unused baby formula containers to return them immediately. But the FDA continues to recommend that parents and caregivers stop using the product and throw away the baby formula in this recall.
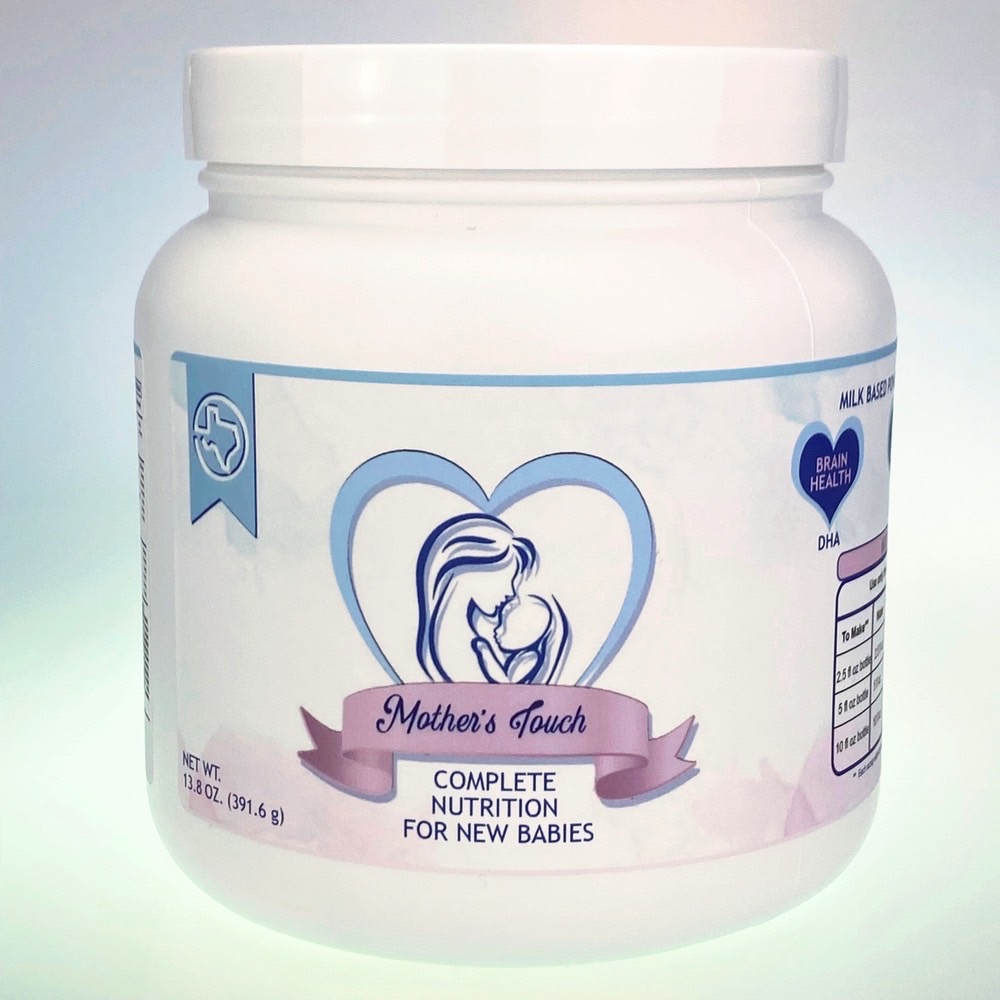
What’s the problem with the Mother’s Touch baby formula?
The FDA explained in the initial warning that the Mother’s Touch product isn’t manufactured in compliance with the FDA’s infant formula regulations.
The company did not test the product to determine whether it meets the nutrient requirements for baby formula. Moreover, the product contains label claims for seven nutrients that do not meet the nutrient requirements for baby formula.
Specifically, the label says that the product contains nutrient amounts below the minimum levels required for protein, linoleic acid, calcium, sodium, potassium, and chloride. Furthermore, the product had more iron than the maximum allowed.
As a result, the formula has the potential to cause nutrient deficiencies or iron toxicity in infants.
On top of that, the formula was not tested for the presence of dangerous bacteria like Cronobacter. That bacteria triggered the Abbott baby formula recall earlier this year.

What you should do
The FDA initially advised parents and caregivers to stop using the Mother’s Touch baby formula. The agency maintains its recommendation now that a voluntary recall is in place.
Parents worried about the development of their infants after consuming the baby formula should seek help from their healthcare providers.
Moreover, the FDA offers contact information that parents can use to report complaints or adverse events. See the FDA’s warning announcement at this link.
As for the actual Mother’s Touch baby formula recall, there’s no FDA press release for it. And there’s no announcement on the company’s website either.








