Blaine Labs Company issued a recall for one lot of RevitaDerm Wound Care Gel. The company initiated the recall after discovering the presence of a bacteria (Bacillus cereus) in a bottle from that lot. If you have any wound care gels at home, you should check your medicine cabinet and ensure your supply doesn’t come from the RevitaDerm lot that’s unsafe to use.
The RevitaDerm Wound Care Gel recall
Blain Labs recently announced the wound care gel recall in conjunction with the US Food and Drug Administration (FDA).
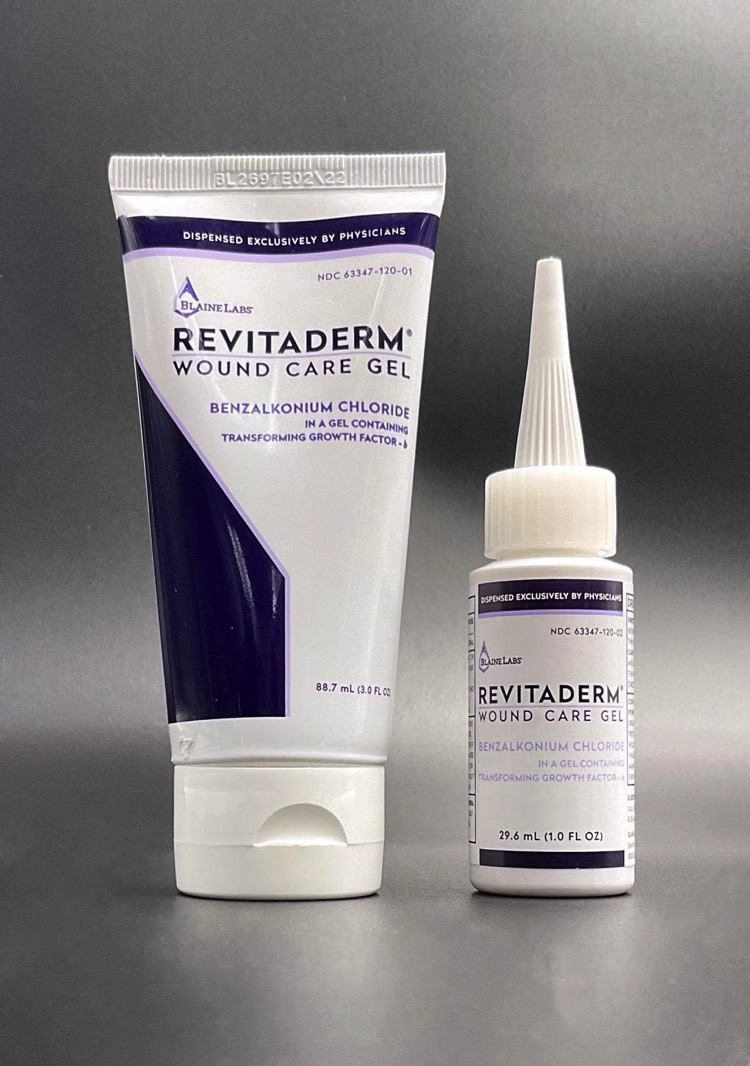
RevitaDerm Wound Care Gel is a drug you can use as an antimicrobial on cuts and scrapes. The product comes in 1.0-ounce bottles and 3.0-ounce tubes, as seen below. They have “RevitaDerm Wound Care Gel” printed on the front, as well as a Drug Facts label on the back.
The company distributed the drug to 61 physicians in 17 states last year. But Blain Labs only recalled a single lot. You should be looking for “BL 2844” on the package. The expiration date is 02/19/2023, which means the product has a long shelf life and could still be in homes around the country.
The Bacillus cereus infection risk
The announcement for this wound care gel recall explains that patients who use the product risk developing an infection with the Bacillus cereus bacteria. Applying the contaminated product to a wound would result in a potentially dangerous infection of the skin and soft tissue.
People who aren’t immunocompromised can expect less severe infections that typically respond to treatment. But the Bacillus cereus can be more dangerous to immunocompromised patients and preterm babies. The bacteria can cause “life-threatening, invasive infections including wound and blood infections, sepsis, pneumonia, and meningitis.”
Blain Labs says that it has not received any complaints or reports of adverse events connected to the wound care gel that’s being recalled.
What you should do
If you have RevitaDerm Wound Care Gel at home, make sure you check that it’s not part of the recall. Any products that aren’t from the BL 2844 lot are still safe to use. If you own medicine from the recalled lot, you should stop using it right now and contact your doctor. Either discard the drug or return it to the doctor who prescribed the treatment.
If you think you’ve experienced any adverse effects related to the recalled gel, you should also inform your doctor.
As always with recalls, make sure you read the full press release, which is available on the FDA website. The announcement contains contact information for Blaine Labs. It also has details on reporting adverse reactions and quality problems to the FDA’s MedWatch Adverse Event Reporting program.
Finally, you should check out two other drug recalls that involve potential contamination with microbes: The Senna Syrup recall and the Rompe Pecho recall.