Urgent Medicine Recall: A Serious Dosage Mistake Can Lead To Overdose And Death
People who treat chronic pain with Morphine Sulphate should be aware of a recall involving two versions of the drug. Bryant Ranch Prepack is recalling two lots of Morphine Sulphate because they're mislabelled, with the bottles showing incorrect doses for the pills.
As a result, people can experience severe side effects by taking the wrong dose of the medicine, including overdose and death.
Morphine Sulphate recall
Bryant Ranch Prepack announced the recall this week. Then, the US Food and Drug Administration (FDA) publishing the press release on Thursday.
The action involves two lots of Morphine Sulphate with 10 bottles per lot. Each bottle contains 100 tablets. The products are Morphine Sulfate 30 mg Extended-Release, and Morphine Sulfate 60 mg Extended-Release.
The problem with the drugs is that they have incorrect labeling.
Bryant Ranch's recalled Morphine Sulfate 60 mg Extended-Release bottles contain Morphine Sulfate 30 mg pills. Similarly, Morphine Sulfate 30 mg Extended-Release bottles may have Morphine Sulfate 60 mg tablets inside.
Since the drug is so strong, this is a dangerous mistake.
The following identifiers should help you determine whether the Morphine Sulphate recall affects you:
- Morphine Sulfate Extended-Release Tablets 30mg – NDC: 63629-1088-01; Lot: 179642; Expiration: 11/30/2023
- Morphine Sulfate Extended-Release Tablets 60mg – NDC: 63629-1089-01; Lot: 179643; Expiration: 08/31/2023
Moreover, you should know the two drug types have different designs.
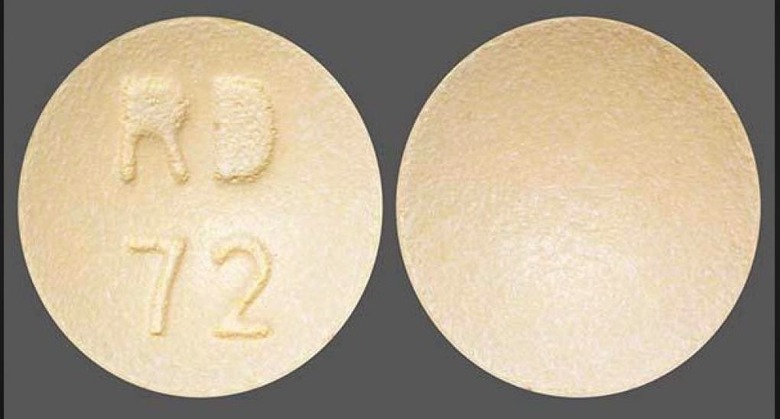
The 30mg are round, purple, film-coated tablets with "RD" and "71" on one side. The other side is plain. Meanwhile, the 60mg tablets are round and light orange in color. They're also film-coated and have "RD" and "72" printed on one side.
What happens if you take the wrong dosage?
Consuming drugs with the wrong dosage can always lead to adverse reactions.
If you have any bottles from the Morphine Sulphate recall, you risk experiencing various side effects. Or you may have already felt them.
Patients prescribed the 30mg dose would have received 60mg pills. This can lead to accidental overdose and death.
Additionally, patients who have to take the 60mg dose would have only received 30mg pills. This can cause withdrawal symptoms. And people may continue to experience pain, as the lower dosage might not be enough to treat it.
"Patients prescribed the 30 mg dose who receive the 60 mg dose could be at risk for overdose and death. Patients prescribed the 60 mg dose who receive the 30 mg dose may experience withdrawal and untreated pain if the dose given is too low," the official recall alert reads.
This recall is extremely urgent
Bryant Ranch Prepack says it has not received any reports of adverse events related to the recall. But consumers who received Morphine Sulphate pills from the recalled lots risk developing side effects.
The company is notifying distributors and customers by email, phone, and letter. It says it is arranging for the return of the products listed in the recall.
People who rely on this drug to treat pain should consider reaching out to their doctors to ensure they get adequate treatment. You should also seek medical guidance if you've experienced unexplained symptoms after using these drugs.
Finally, you should check out the full Morphine Sulphate recall announcement at this link. It contains contact information for the company, links to other FDA resources, and more product images.