- Six coronavirus vaccine candidates have been determined to be the likeliest to succeed, a Morgan Stanley analyst said in a recent note to investors.
- The list of candidates includes a few familiar names, like Moderna, Oxford, and BioNTech. These labs are already conducting promising human trials, and the drugs could be approved later this year.
- Other vaccine candidates from Johnson & Johnson and Sanofi could be ready by the end of 2021.
- Visit BGR’s homepage for more stories.
There’s no cure for the novel coronavirus right now, but significant progress is being made. Physicians treating COVID-19 patients have been able to discover all sorts of unusual symptoms and come up with treatments that can speed up recovery and reduce complications. Similarly, vaccine research looks incredibly promising.
We have no idea if a vaccine will be able to generate the desired immune response and block infection, but more than 100 teams came forward with ideas on how to stop the virus. As many as eight of them are already in various phases of clinical trials, and at least a couple of them could be approved for emergency use as soon as this fall. Of all of them, analysts at Morgan Stanley think that six of the existing candidates are the most likely to succeed.
Even if a vaccine is ready as soon as this fall, it will be years before it is widely available. As Bill Gates explained in his recent remarks, manufacturing and distributing up to 7 billion doses of a COVID-19 vaccine will require resources, time, as well as a strategy to ensure that the entire world has access to the drug. Gates said he expected more than one candidate to be approved for COVID-19 of the several his foundation has been funding.
Dr. Anthony Fauci said recently that he’s hopeful the first vaccine will be ready as soon as early 2021, but explained in a paper that a single candidate won’t be enough for the entire world.
The fact that as many as six vaccines are seen as likely to succeed is definitely good news. But, as before, there is no guarantee these drugs will perform on humans as well as they did on animals or in labs. Vaccines have to be both effective and safe, which is why so many human trial phases are required.
Analyst Matthew Harrison said in a note seen by Benzinga that millions of doses of the vaccine could be available this fall, and over a billion next year. He singled out six drugs as the most promising, including four that are already in clinical tests, and two candidates that are still in the preclinical phase.
The four vaccines that are being tested right now aren’t a surprise. We’re looking at Moderna’s mRNA vaccine, which was the first to reach human trials, and the first to report positive results. Then we have the Oxford vaccine that performed well on monkeys and is progressing with promising results on humans. BioNTech’s genetic vaccine also started tests in Germany and the US in recent weeks, in partnership with Pfizer. Then there’s the CanSino trial in China and in Canada. The analyst believes all four could be available later this year.
In the first half of 2021, Johnson & Johnson’s vaccine candidate might be available, while the Sanofi-GlaxoSmithKline vaccine candidate could be approved by late 2021. The following table lists all of these potential COVID-19 vaccines, highlighting the vaccine platforms, current status, and initial manufacturing capacity:
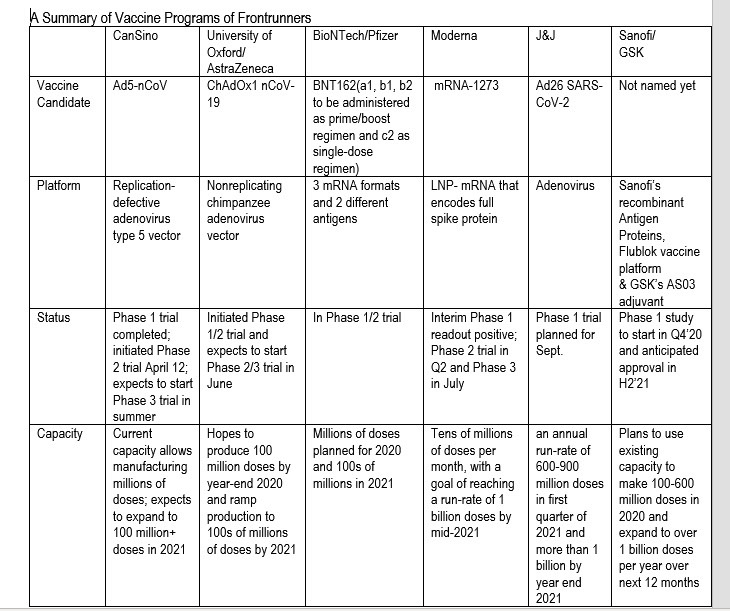
These are estimates based on the current progress for all six vaccine candidates, and things could change in the coming months. Morgan Stanley acknowledged that clinical data is still needed, and even when it is available, it might be difficult to judge. The analyst said that neutralizing antibody levels obtained in volunteers who were inoculated will be compared with animal models and antibody titers achieved by COVID-19 survivors.
The analyst also said that NIAID might be working on establishing neutralizing antibodies titers thresholds that could offer protection against the disease. That data could be released before the first vaccines are out.
The research note said the coronavirus pandemic market could be worth $10 billion to $30 billion, while the endemic opportunity sits at $2 billion to $25 billion.