- Coronavirus vaccine research has advanced at rapid speed, providing promising early results that suggest such drugs could provide immunity and protection against severe illness.
- However, recent COVID-19 vaccine controversies may have eroded the public’s trust in the new drugs.
- Researchers who have been following COVID-19 vaccine development published a paper that explains everything that happened so far this year.
At the start of the novel coronavirus pandemic, health experts including Dr. Anthony Fauci said that a vaccine might be ready within 12 to 18 months. That sounded great at the time, suggesting that scientists were fairly confident of the chances of success. The months that followed saw more than 100 coronavirus vaccines enter trials, with some of the most promising ones having now advanced to Phase 3 clinical trials.
The summer brought us a massive surge in COVID-19 cases in the US, Brazil, India, Russia, and other countries around the world, showing the virus would not slow down in warmer months like the flu. Access to an effective vaccine seemed even more crucial, especially as some coronavirus research said immunity might not last that long. The first Phase 1 and Phase 2 results came in for several of the advanced vaccine candidates, delivering promising news. The chances of success seemed high considering that the number of experimental vaccine candidates jumped well over the 100 candidates that were registered in the first few months of the pandemic. With all those different ideas, surely some of them might work. And the more effective vaccines we have, the higher the chances of meeting global demand. The early results also showed that we could realistically expect at least one vaccine candidate to clear the final stage of human trials and be approved by the FDA for emergency use in late 2020.
But just as vaccine research seemed to exceed expectations, surveys started showing that not everyone was thrilled at the prospect of getting a vaccine. A few months ago, one-third of Americans who were surveyed said they would not get a vaccine. That’s a significant percentage, as resistance to vaccines might compromise heard immunity efforts. The most recent poll shows that as many as two-thirds of Americans will not get a COVID-19 vaccine, at least not initially. It’s not that the anti-vaccination movement has gained more traction in the midst of a massive pandemic, but recent controversies may have had a direct impact on the population’s enthusiasm for the drug. If you’re worried about the coronavirus vaccine development though, this is the research paper you need to check out.
A couple of recent vaccine controversies may explain why some people are worried about COVID-19 vaccination. It all has to do with the speed of the research and development of vaccines, seen through the recent moves that various countries have made. Russia approved its vaccine for emergency use without showing the world any scientific data to suggest that it actually works. China published plenty of research that shows several drugs do induce the desired immune response without severe side-effects, but the country has also approved three vaccines for emergency use before Phase 3 trials. Then reports came in saying that the Trump administration might pressure the FDA to rush its first coronavirus vaccine authorization so it coincides with the presidential election in November.
You don’t have to be a die-hard anti-vaxxer to witness all these developments and start worrying about the safety and efficacy of vaccines. Going through the available literature that details COVID-19 vaccine development might be cumbersome and annoying, but that’s one way to alleviate your concerns. You’ll find research on all the Phase 3 drugs, whether it’s Moderna, Pfizer/BioNTech, AstraZeneca/Oxford, or the three similarly advanced vaccine trials from China. Other research is available for vaccines that are in preclinical or Phase 1/2 trials. Novavax, Johnson & Johnson, and even Russia have published results for their drugs.
But if there’s a single coronavirus vaccine-related paper that you should read, it should be the Evolution of the COVID-19 vaccine development landscape that was published in Nature on Friday.
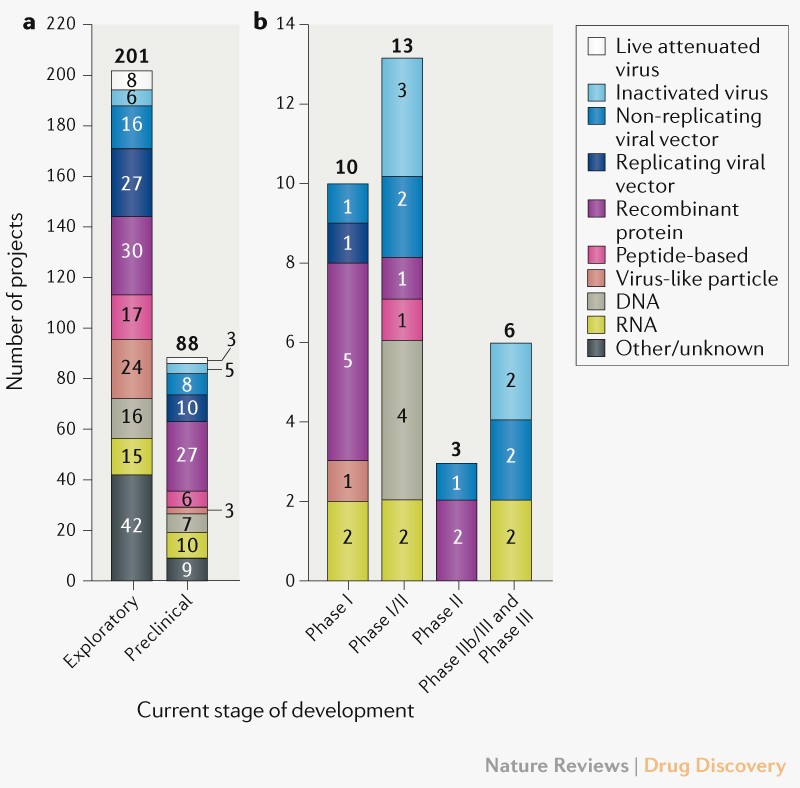
The paper provides all the data about COVID-19 vaccine work so far, giving you a quick run-through of what’s happening in the industry right now. While the article can’t provide any sort of guarantee that these vaccines will work, it still offers a hopeful outlook that should counter all the other controversies where politics are involved. The researchers also provide input on what should be expected from COVID-19 vaccine development, and what to look for in upcoming vaccine data.
The researchers who compiled the data explain that as of September 2nd, 321 vaccine candidates are in development, compared to 112 in April. Of those, 32 vaccine candidates are in clinical trials and they will enroll more than 280,000 participants from 470 sites in 34 different countries. That’s to say the data will be as robust as possible for drugs that reach Phase 3 and finis the research. The studies that follow will provide clear information about the safety and efficacy of these drugs.
The paper also explains the various vaccine technologies that have been used to develop COVID-19 drugs. We’re looking at “a broad range of technology platforms, including both traditional and novel approaches.” The majority of these vaccines target the virus’s spike protein that binds to human cells, but other candidates go for different targets. In other words, not only do we have more than 300 vaccines in development, but the teams working on them are testing various ways of blocking the virus from infecting cells and/or reducing the severity of an infection. This increases the chances that we’ll have at least some effective vaccines in the near future, and that’s even if the current Phase 3 drugs fail.
The researchers also note that “encouraging antibody and T cell responses have been reported for vaccines based on several of the different platforms being used,” but acknowledge that it’s too early to assess their relative potential. They also say that the presence of both of these components of the immune system may be required to provide protection against COVID-19, so vaccines should induce both responses. Various vaccines have already proven that they can “manufacture” neutralizing antibodies and T cells.
According to the paper, 11 drugs come from Chinese organizations and seven are supported by Operation Warp Speed in the US. The US government’s endeavor aims to provide 300 million vaccine doses by January 2021, with more than $10 billion already invested to advance development. Eight of those drugs have received funding from the Coalition for Epidemic Preparedness Innovations (CEPI) and are included in the COVAX portfolio. That’s a collaboration between CEPI, the Gavi Alliance, and the World Health Organization (WHO).
The paper also looks at the actual clinical development process, explaining the challenge of manufacturing a vaccine during an ongoing pandemic fueled by a novel pathogen that needs further studying. Furthermore, the scientists explain what the endpoints, or goals, for the vaccine candidates should be. They explain that the goal of vaccination trials should not be asymptomatic infections, as that sort of result might not be measured adequately. An endpoint requiring signs or symptoms of pneumonia may prove whether the drug works. That’s to say, if volunteers get infected but do not develop pneumonia that can lead to severe respiratory complications, multiple organ failure, and death, then the vaccine might be deemed effective.
Finally, the paper notes that even though uncertainties remain about COVID-19 vaccine research, the first Phase 3 trial results should provide “valuable insights for the field and inform ongoing and future development activities aimed not only at controlling the current global pandemic but also for effective long-term immunization strategies against the disease.”
The full paper is available in Nature.








