- Doctors from China conducted a Phase 2 trial for a triple-drug therapy in COVID-19 cases, proving that the three medicines can speed up recovery in mild to moderate cases.
- Coronavirus patients who were given the three drugs being asymptomatic faster than the control group and cleared the virus in an average time of one week.
- The control group that was on an HIV drug that is also being tested for COVID-19 needed 12 days, on average, to test negative for the novel coronavirus.
- Visit BGR’s homepage for more stories.
The novel coronavirus had infected more than 4 million people worldwide as of Saturday morning, of which nearly 280,000 died fighting the disease. Some 2.4 million cases remain active around the world, but the number is likely far higher than that. Asymptomatic and mild COVID-19 patients can go undetected in many countries where there aren’t enough tests to go around. But the world has made tremendous progress at treating the disease and it’s going to be even easier to treat patients in the years to come, as we wait for viable vaccines to become widely available.
Remdesivir can reduce the recovery time from 15 days to 11 days according to a study that included more than 1,000 people, and is considered standard therapy for COVID-19. There’s not enough of the drug to go around though, so remdesivir alone won’t be enough. The drug can’t reduce mortality rates either, so additional medicines will be required. Blood thinners can reduce COVID-19 complications and death risk, as a different study has shown. Men given a specific type of prostate cancer treatment might also experience a milder COVID-19 case.
These are all COVID-19 therapy developments that have been detailed in the past few days. Now, we have gotten word of another novel coronavirus treatment that works, a triple-drug combo that can dramatically speed up recovery in mild and moderate cases.
Published in The Lancet journal, the study details a Phase 2 randomized trial at Hong Kong University for three drugs, including interferon beta-1b, lopinavir-ritonavir, and ribavirin.
If the lopinavir-ritonavir combo sounds familiar, that’s because it’s an antiviral used for HIV patients and it’s usually sold under the name Kaletra. The drug has been used in other COVID-19 clinical trials. Ribavirin is also an antiviral that can be used to treat Hepatitis C, among other diseases. Interferon beta-1b is an immunity booster that can be used to treat multiple sclerosis.
The Hong Kong doctors split a group of 127 COVID-19 patients into two cohorts: 86 were assigned to the triple-drug therapy while 41 were included in the control group that was treated with Kaletra. These were all patients who developed mild to moderate cases of COVID-19. However, some patients were intubated and received ventilation therapy during treatment. All patients recovered, but one person in the control group had to interrupt the therapy after hepatic enzymes tested higher than supposed to.
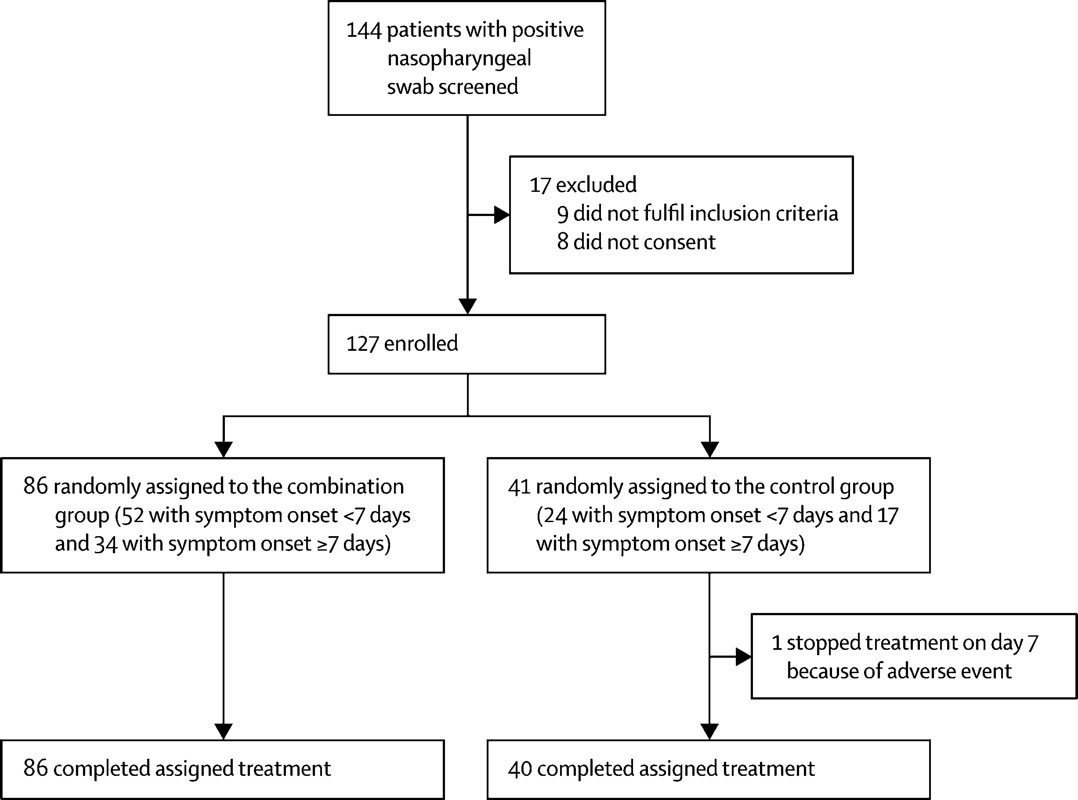
The average number of days from symptom onset to start of the study was 5 days for the triple-drug group, and 4 days for the control. The triple-drug group recovered much faster, testing negative for the novel coronavirus in an average of 7 days. The patients in the control group needed 12 days to clear the virus, on average. Adverse effects included nausea and diarrhea, with no difference between the two groups.
Also interesting is the fact that patients taking the three drugs started feeling better faster. They stopped showing symptoms after an average of 4 days compared to an average of 8 days for the control group.
The study indicates that taken together, interferon beta-1b, lopinavir-ritonavir, and ribavirin can eliminate the virus and significantly speed up recovery. These drugs appear to work even faster than remdesivir. It also suggests that the quicker the treatment is given after a positive diagnosis, the better the outcome for patients. This study doesn’t explain whether the triple-drug combo might also work in more severe cases, though.
“This study is really refreshing because it tells us remdesivir isn’t the only game in town, and maybe there are other options around,” University of California San Francisco’s Dr. Peter Chin-Hong told CNN. “These drugs have a track record of safety.”
“Maybe we can get this when we can’t get the alleged magic bullet,” he said. There is no magic bullet, however. Remdesivir isn’t and shouldn’t be considered a magical cure. Even Dr. Anthoni Fauci warned a few days ago that the next step is pairing remdesivir with a different drug to see if even better results can be obtained.