- Dr. Fauci and other health experts authored a paper that explains what the world has to do to ensure that a viable COVID-19 vaccine reaches the public as fast as possible.
- The scientists explained that a single vaccine candidate will not be enough in the fight against the novel coronavirus, and more drugs will be needed to satisfy the global need for a vaccine.
- The paper also details a public-private partnership called ACTIV “for harmonized clinical trials aims to accelerate licensure and distribution” for COVID-19 vaccines.
- Visit BGR’s homepage for more stories.
The novel coronavirus still rages on and there’s no sign that the infection will go away anytime soon, even if several countries and communities were able to flatten the curve and reduce the number of new COVID-19 cases. The virus is just that contagious, and anyone can get it, even if not everybody will display signs of the infection. To beat this thing, we’ll need two types of treatments in the coming years. We need drugs that can act early against the disease and reduce the risk of complications and death. And then we need vaccines that can prevent the infection and hopefully eradicate it. That’s “vaccines,” plural. Just one drug won’t be enough to inoculate everyone around the world, and we’ll need more than 7 billion doses of vaccines ready in the next few years.
Dr. Anthony Fauci, the director of the National Institute of Allergy and Infectious Diseases, co-authored a paper about the path forward for COVID-19 vaccine research and development. Fauci and other experts call for collaboration, unity, and transparency at the national and global level to expedite the arrival of safe and effective vaccines.
“There is an unprecedented need to manufacture and distribute enough safe and effective vaccine to immunize an extraordinarily large number of individuals in order to protect the entire global community from the continued threat of morbidity and mortality from severe acute respiratory syndrome–coronavirus 2 (SARS-CoV-2),” the paper reads.
Published in Science Mag, the paper has other notable co-authors including the National Institutes of Health director Dr. Francis Collins, and NIH vaccine expert Dr. John Mascola.
The experts explain the daunting task ahead and why manufacturing a viable vaccine for a disease like COVID-19 isn’t easy. It’s not solely that we need to wait for clinical trials to prove the safety and effectiveness of the vaccine candidates. We also have to prepare to manufacture viable compounds at scale and ensure the logistics required to deploy the drugs worldwide.
The paper says the current evolution of the SARS-CoV-2 virus suggests that a vaccine would still work up to a year from now even if the virus continues mutating, which is hopeful news. It also explains the various promising vaccine candidates that have reached different stages of trials in the US and elsewhere, describing the risks and challenges associated with manufacturing each one of them should they prove to be effective at generating the expected immune response.
Among the notable COVID-19 vaccine candidates out there, the authors highlight genetic approaches like the ones from Moderna, BioNTech/Pfizer, and Inovio. They also mention more traditional methods that use viral vectors, like the one coming from the University of Oxford and AstraZeneca.
The paper also tackles the growing demand for challenge trials that involve intentionally infecting volunteers with the novel coronavirus after they’d been given vaccine candidates to test effectiveness faster than regular vaccine research protocols. The WHO has already approved guidelines for such procedures and more than 14,000 people worldwide have expressed interest in undergoing such tests.
“It is encouraging that vaccine development efforts have moved swiftly, and several major vaccine platforms are moving toward clinical evaluation,” the paper reads, adding that “no single vaccine or vaccine platform alone is likely to meet the global need, and so a strategic approach to the multi-pronged endeavor is absolutely critical.
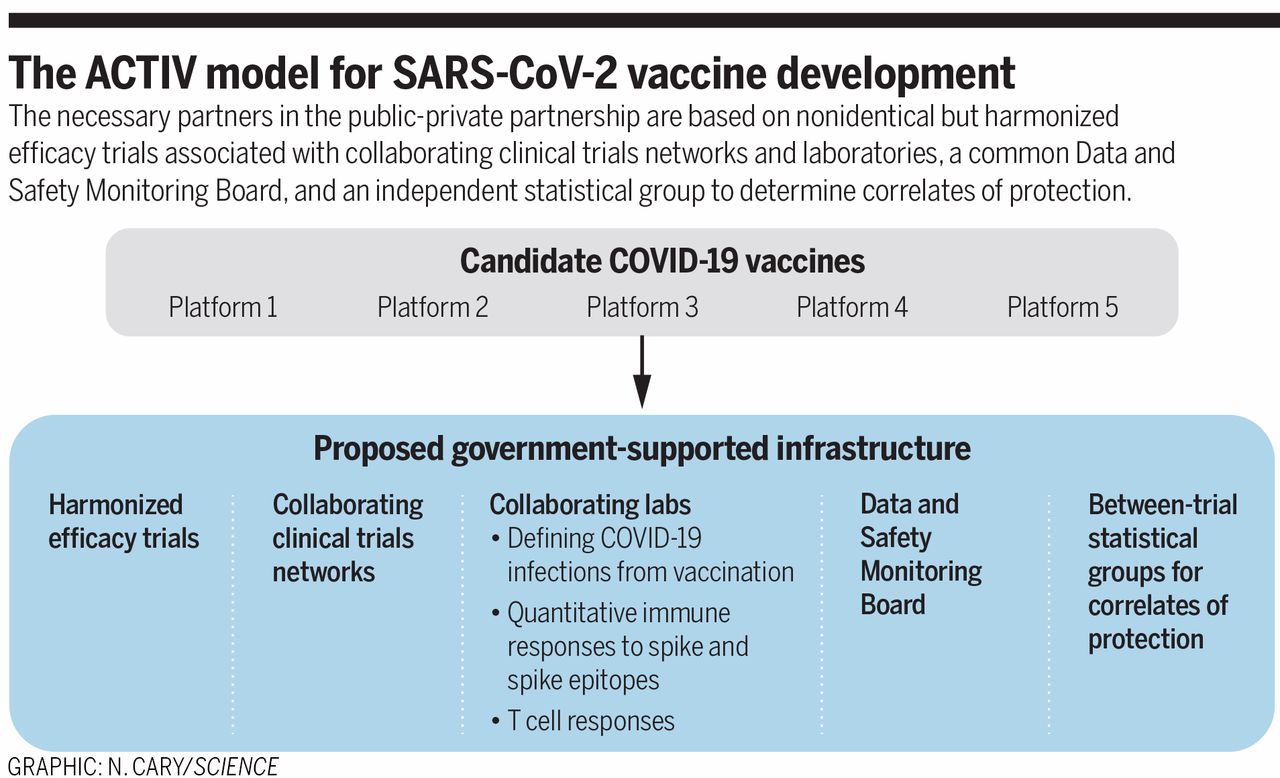
The paper also introduces a new collaborative program started by NIH. Called ACTIV (Accelerating COVID-19 Therapeutic Interventions and Vaccines), a public-private partnership that’s supposed to speed up the development of vaccines. The government proposes increased collaboration and transparency between the various decision factors and companies involved in the multiple stages of COVID-19 vaccine development right now to hasten the actual deployment of a viable drug.
NIH has partnered for ACTIV with the FDA, CDC, the Department of Health and Human Services, Biomedical Advanced Research and Development Authority, Departments of Defense and Veterans Affairs, the European Medicines Agency. Other partners include more than 15 biopharmaceutical companies, philanthropic organizations, representatives from academia, and the Foundation for NIH.
“Cost, distribution system, cold chain requirements, and delivery of widespread coverage are all potential constriction points in the eventual delivery of vaccines to individuals and communities. All of these issues require global cooperation among organizations involved in health care delivery and economics,” the paper notes. “To return to a semblance of previous normality, the development of SARS-CoV-2 vaccines is an absolute necessity. To achieve this goal, all the resources in the public, private, and philanthropic sectors need to participate in a strategic manner. The ACTIV public-private partnership and collaborative harmonized efficacy trials are enabling models to achieve our common goal.”
Bill Gates, who is also at the forefront of COVID-19 vaccine development through his Bill and Melinda Gates Foundation, touched on these various aspects in recent weeks. He explained the need to develop multiple vaccines and fast-tracking plans to mass-produce the candidates that are ultimately selected.
Fauci said a few weeks ago that in a best-case scenario, the first vaccine might be available as soon as early 2021 if everything goes well. In a follow-up interview with National Geographic, Fauci expressed great optimism for Moderna’s mRNA vaccine, which is the most advanced clinical trial right now. But he also said that they want “a lot of shots on goal,” looking forward to four or five candidates that would reach more advanced phases this summer. The good news is that as many as 115 teams were testing distinct coronavirus vaccine candidates as of mid-April, so the hopes are high for at least one of them to work.