- The UK government announced that the breakthrough Oxford coronavirus vaccine candidate is making progress in its human trials.
- If effective and safe, 30 million doses of the ChAdOx1 nCoV-19 vaccine candidates could be ready for use in the UK by September.
- The UK, Oxford, and AstraZeneca will also mass-produce the drug for other countries once it obtains regulatory approval.
- Visit BGR’s homepage for more stories.
Several coronavirus vaccine candidates are in various phases of testing after having performed well in lab trials. Phase 1 and Phase 2 trials are running in multiple countries, including China, Germany, the UK, and the US, using multiple types of vaccine technology. Some of the vaccine candidates might be approved for emergency use as soon as this fall, including a breakthrough drug from Oxford that already works on monkeys.
If the vaccine is safe and effective on humans, as many as 30 million doses might be ready by September in Britain, of the initial 100 million capacity that AstraZeneca has promised.
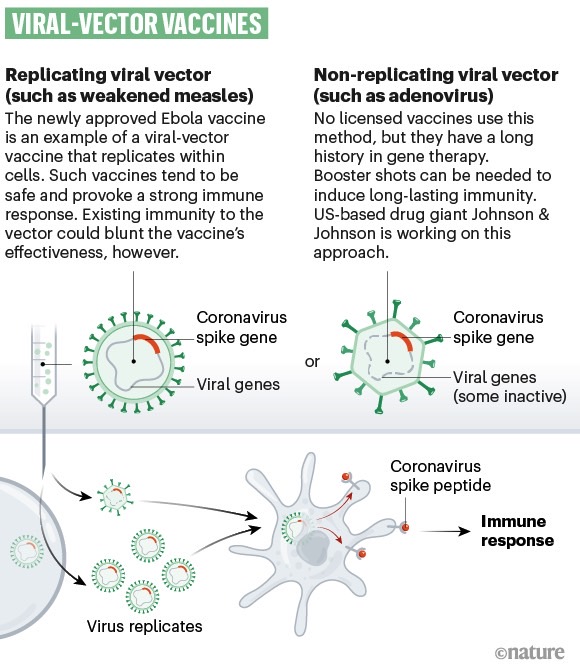
ChAdOx1 nCoV-19 is the name of the Oxford vaccine that uses a weakened chimp virus engineered to carry the spike protein of the SARS-CoV-2 virus. The idea is to inoculate patients with this medicine to trigger an immune response that would generate antibodies to that spike protein. Upon an encounter with the actual novel coronavirus, the body would already have the means to block that protein from binding to human cells.
Researchers already explained that the six monkeys immunized with the vaccine candidate have all developed the antibodies that blocked SARS-CoV-2 from entering cells and multiplying. The study also proved that the drug was safe for the animals, as it didn’t cause an enhanced COVID-19 case in the subjects.
Human trials started in mid-April, thanks to the promising data obtained in the monkey trials, and Oxford partnered with AstraZeneca on the development of the drug. Britain’s Business Secretary Alok Sharma told reporters on Sunday that plans are in place to roll out the COVID-19 vaccine to 30 million people by September, Sky News reports.
Sharma said that Oxford’s study is “progressing well,” but he pointed out there’s no guarantee that the vaccine will work on humans.
“The first clinical trial of the Oxford vaccine is progressing well with all phase one participants having received their vaccine dose on schedule earlier this week,” he said. “The speed at which Oxford University has designed and organized these complex trials is genuinely unprecedented.” He also added that AstraZeneca had finalized a “global licensing agreement” with Oxford University with government support.
“This means that if the vaccine is successful, AstraZeneca will work to make 30 million doses available by September for the UK as part of an agreement for over 100 million doses in total.”
If the vaccine is successful, the 30 million doses would be enough to immunize nearly half of Britain’s population. As of Monday morning, the UK had registered nearly 244,000 COVID-19 cases, one of the highest figures in Europe, and 34,636 deaths. Sharma explained that the UK will be the first to get access to Oxford’s drug, but the government would ensure that “we’re able to make the vaccine available to developing countries at the lowest possible cost.”
Finally, the official said the UK’s first manufacturing innovation center is expected to open in summer 2021, a year ahead of plan. Once finalized, the center will be able to produce enough vaccine doses to serve the UK’s population within six months.
Aside from vaccines, six drugs have entered clinical trials in the UK, he said, explaining that vaccines might not be successful. That’s why it’s necessary to continue looking for other therapies. Imperial College London is also “making good progress” on a vaccine candidate, with Phase 1 trials to start by mid-June.








