- Researchers have another coronavirus drug that seems to work in severe COVID-19 cases.
- A limited study of a monoclonal antibody drug called narsoplimab showed that all the patients who received it were taken off ventilators and survived eventually ended up being discharged from the hospital.
- Narsoplimab doesn’t block the coronavirus’s ability to infect cells, but it prevents the damage that occurs inside the blood vessels of COVID-19 patients, which can affect various organs and lead to death.
We may be wrong to expect a miracle cure for COVID-19. That’s because it might not be just one breakthrough drug that delivers the “miracle,” which is the prevention of life-threatening symptoms. We may end up with several new drugs for COVID-19 that would work independently or in tandem with others to save the lives of patients who develop complications.
Recent studies showed that several therapies can reduce COVID-19 mortality to some degree. But neither remdesivir nor dexamethasone can prevent deaths completely. This is where narsoplimab may come to the rescue, a monoclonal antibody drug that’s not supposed to block the coronavirus from infecting cells like other monoclonal therapies being tested for COVID-19. Instead, this drug protects the integrity of blood vessels and helps prevent clotting. Narsoplimab saved the lives of every one of the critical COVID-19 patients who were included in a limited trial in Bergamo, Italy, one of the early epicenters of the European COVID-19 epidemic.
Made by Omeros, narsoplimab was initially devised for other medical conditions that cause damage to the blood vessels, but doctors involved with previous clinical testing thought the drug might also work on COVID-19 cases. Dr. Alessandro Rambaldi from the Papa Giovanni XXIII hospital in Bergamo was involved in a stem-cell trial for narsoplimab before COVID-19, Omeros explained in a press release. The doctor asked Omeros to offer the drug to six COVID-19 patients in critical condition, who needed mechanical ventilators to breathe.
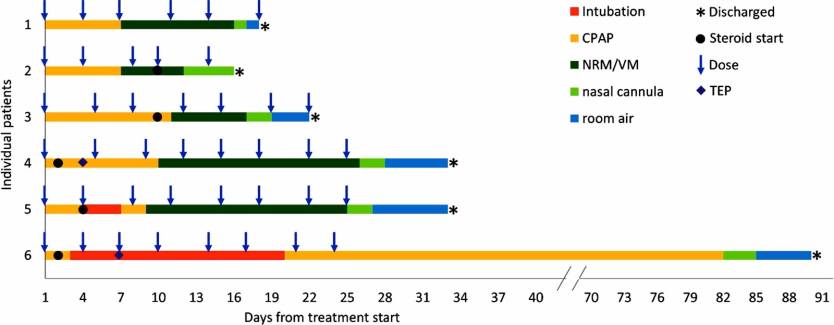
These patients developed Acute Respiratory Distress Syndrome (ARDS), one of the COVID-19 complications that can lead to death. The patients were aged 47 to 63, all had comorbidities, and five of them were men. The researchers administered narsoplimab 48 hours after mechanical ventilation had started, and then all the patients received the drug twice a week for two to four weeks.
All the patients recovered and survived COVID-19, but two of them were hospitalized for about a month after narsoplimab treatment began. Another spent some three months in the hospital after the first dose.
“Endothelial injury appears to be caused by direct viral infection” in COVID-19, the researchers wrote in the paper, and that damage can be measured with the help of several parameters. The doctors measured those parameters, which proved the drug reduced the cellular damage inside the blood vessels. Narsoplimab also prevents blood clotting, the researchers say, and it might prevent so-called cytokine storms, the overreaction of a patient’s immune system that can be fatal.
The temporal improvement of IL-6 and IL-8 with narsoplimab treatment suggests that lectin pathway activation may precede cytokine elevation in COVID-19 and that lectin pathway inhibition has a beneficial effect on the cytokine storm described in patients with COVID-19 infection
The researchers say the drug was well tolerated and there were no side-effects. The study also reveals that five of the six patients received steroids at the hospital, with two of them getting the drug after their condition improved significantly (patients 2 and 3 in the graphic above).
One downside of the study is that it wasn’t a double-blind, placebo-controlled, randomized trial. But the researchers compared the six patients with two control groups that met similar criteria and characteristics, which showed mortality rates of 32% and 53%.
“The patients that we treated with narsoplimab were critically ill, and the uniformly successful outcomes were truly impressive,” Rambaldi said. “Also of importance in this terribly sick population studied, the drug was well tolerated, showing no adverse effects. The pathophysiology of COVID-19 appears to be consistent with that of stem cell transplant-associated TMA, and the mechanism of the lectin pathway inhibitor narsoplimab looks to be well suited to treat the often-lethal manifestations of both disorders. The outcomes in these six patients provide further evidence of the potential role of narsoplimab in treating diseases caused by endothelial damage.”
The drug is under review for federal approval, The Seattle Times reports, and it’s considered for federal support under the Operation Warp Speed initiative.
More research will be required before the drug can be widely used to treat severe COVID-19 cases. If the drug can indeed deliver the same response in most patients who develop complications, it could potentially save a lot of lives. That said, it’s unclear how easy it is to manufacture or how much it will cost.
The Omeros study will be published in the peer-reviewed journal Immunology and is available in pre-print form at this link.