- A company from Canada working on one of several coronavirus vaccines in development will start human trials this summer.
- The drug is a virus-like protein that can deliver an immune response without infecting people with the COVID-19 disease.
- The key ingredient in the vaccine is being grown inside a plant rather than in a laboratory, before being turned into a drug.
- Visit BGR’s homepage for more stories.
The novel coronavirus will be with us for several years at least, experts say, warning that COVID-19 might never be eradicated. A vaccine is our best hope of reaching the level of immunity that can prevent severe outbreaks. It’ll take years to get there even if the first vaccines are ready to be deployed as soon as late 2020 or early next year. It’s not enough to make a vaccine that’s both effective and safe, we also have to find ways to manufacture billions of doses as fast as possible and ship them all over the world. Dr. Anthony Fauci and his colleagues already explained the challenges ahead in a paper on the matter, and Bill Gates provided even more details about how vaccine research and development will move forward.
The good news in all of this is that we have more than 100 vaccines in development, which means scientists have various ideas about how to prevent COVID-19. Some of them have already begun testing, and the preliminary results will be ready soon. Others are getting ready for human trials, including a company in Canada that has an unusual way to create the active substance in the vaccine: The company is growing it in a plant.
Called Nicotiana benthamiana, the plant is known as benth or benthi and it’s related to tobacco. However, the vaccine research has nothing to do with tobacco or smoking.

Researchers from Canadian biopharmaceutical company Medicago announced on Thursday that the vaccine candidate that’s been in development for several months has shown its effectiveness in mice. The project will move to human clinical trials this summer, with Phase 2 expected to start in late 2020.
“These positive results are pivotal to initiate a clinical study in healthy volunteers,” Medicago VP Nathalie Landry said in a statement. “Once results from a second ‘boost’ dose are available, Medicago will submit a clinical trial application to Health Canada and an investigational new drug submission with the FDA in the United States to allow for the initiation of human clinical trials this summer.”
The company produced a virus-like particle (VLP) in March, some 20 days after receiving the SARS-CoV-2 samples. “We were able to take the sequences that we thought would be most interesting, that targeted the spike protein, which is probably the most important viral protein for causing infection,” medical officer for Medicago Brian Ward told Yahoo News Canada.
The spike protein is the novel coronavirus’s key component that can bind to human cell receptors so the virus can replicate itself.
“It faithfully reproduced the viral spike protein in a way that…maintained that spike protein in a form that was likely to cause the immune system to react in a good way, to produce antibodies that would neutralize the real virus and cellular immunity that would help clear the virus from infected cells.”
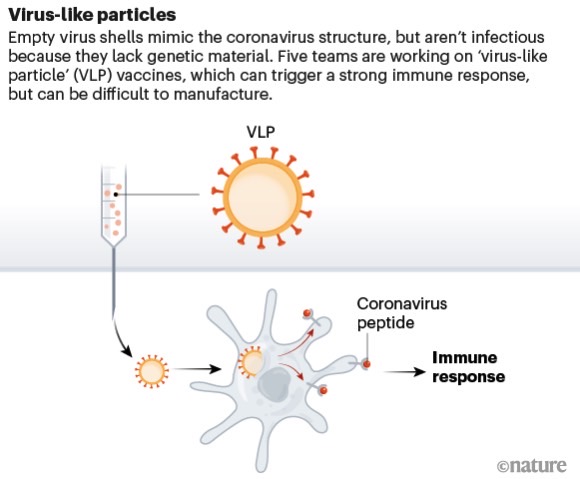
The VLP will trick the immune system into thinking that it’s dealing with a virus so that it triggers an immune system response that should deliver protection against an actual encounter with SARS-CoV-2 in the future. Instead of growing the VLP inside a stainless steel tank with a cell that produces the protein, the scientists are using the plant for the same purpose.
“You can generate huge amounts of what we call biomass, just basically the green leaves, that then go into a relatively small, if you will, pharmaceutical manufacturing set of rooms and what comes out the other side is a highly purified virus-like particle,” Ward said. Thanks to this approach, the company could scale up production by expanding the size of greenhouses where these plants grow.
Using its facilities in Quebec and North Carolina, Medicago could produce up to 120 million doses by 2022. A larger facility in Quebec will be able to produce 1 billion doses of COVID-19 vaccines per year once it’s completed in 2023. ”Millions of doses could be ready by the end of the year as needed,” assuming the vaccine passes regulatory approvals. These are just estimations, however, as the proper dosage for humans hasn’t been determined.
We’ve heard before that some vaccines could be ready for use as early as this fall or by the end of 2020. But they won’t be available to the general population if approved for emergency use. Instead, doctors and other groups of people working in the front lines would be inoculated first.








