- Coronavirus vaccine research is advancing at a fast pace, but that might not be enough for some lawmakers and scientists.
- There’s increasing support for a different type of COVID-19 vaccine clinical trial, one that involves purposely infecting young, healthy volunteers with the novel virus after vaccine inoculations.
- Challenge trials are risky for diseases that have no cure like COVID-19, as there’s no guarantee the young patients will survive the infection.
- Visit BGR’s homepage for more stories.
A vaccine for the novel coronavirus will be the kind of drug that can help eradicate the virus for good. A vaccine would prevent infection and the more people get it, the less likely it is for the virus to spread. The good news is that some 77 vaccines are already in testing, including six that have reached at least phase I of human trials. Also good is the fact that the virus isn’t showing significant mutations that would hinder vaccine efficacy. However, the first COVID-19 vaccines won’t be ready for mass inoculations for up to 18 months. Some of them could be deployed as soon as this fall, but only specific categories of patients will be allowed to get one, including healthcare workers who constantly exposed to the virus.
The vaccines can’t come sooner than that because authorities need to make sure they’re safe, not just effective against the vaccine. The last thing we need to treat COVID-19 is a vaccine that can cause unexpected side-effects. You’d never hear the end of it from anti-vaxxers, not to mention that such an event might delay other vaccine work. But there is an idea to speed up coronavirus research that’s both bold and dangerous.
Some lawmakers and scientists believe that informed volunteers could be given vaccine candidates and then the SARS-CoV-2 virus to see if the vaccine is effective at generating an immune response and preventing infection. This type of study is called “challenge trial,” and it has been done before, The Hill explains.
Challenge trails can be approved for diseases that already have cures aside from a potential vaccine, such as malaria. The problem with COVID-19 is that researchers would have to infect a few hundred young, healthy volunteers with the virus and only some of them would be given the vaccine. Others would receive a placebo drug so that the results can be compared.
Such a study would require the isolation of the patients before applying the vaccine to ensure that nobody already has the disease. Then they’d have to be isolated while the vaccine is administered and during their COVID-19 convalescence. Scientists will have to perform routine tests and observe the evolution of each patient.
The problem with the whole idea is that COVID-19 can kill young, healthy patients as well as older people, and there’s no treatment for it. Even if patients agree to take part in such a study fully knowing what the implications are, it could end up being a death sentence. Also, there’s no guarantee the vaccine will be effective.
A website called 1 Day Sooner is already up in support of vaccine challenge trials, and nearly 3,500 people from 52 countries have signed up for it. The site offers the following comparison between traditional vaccine research and challenge trials:
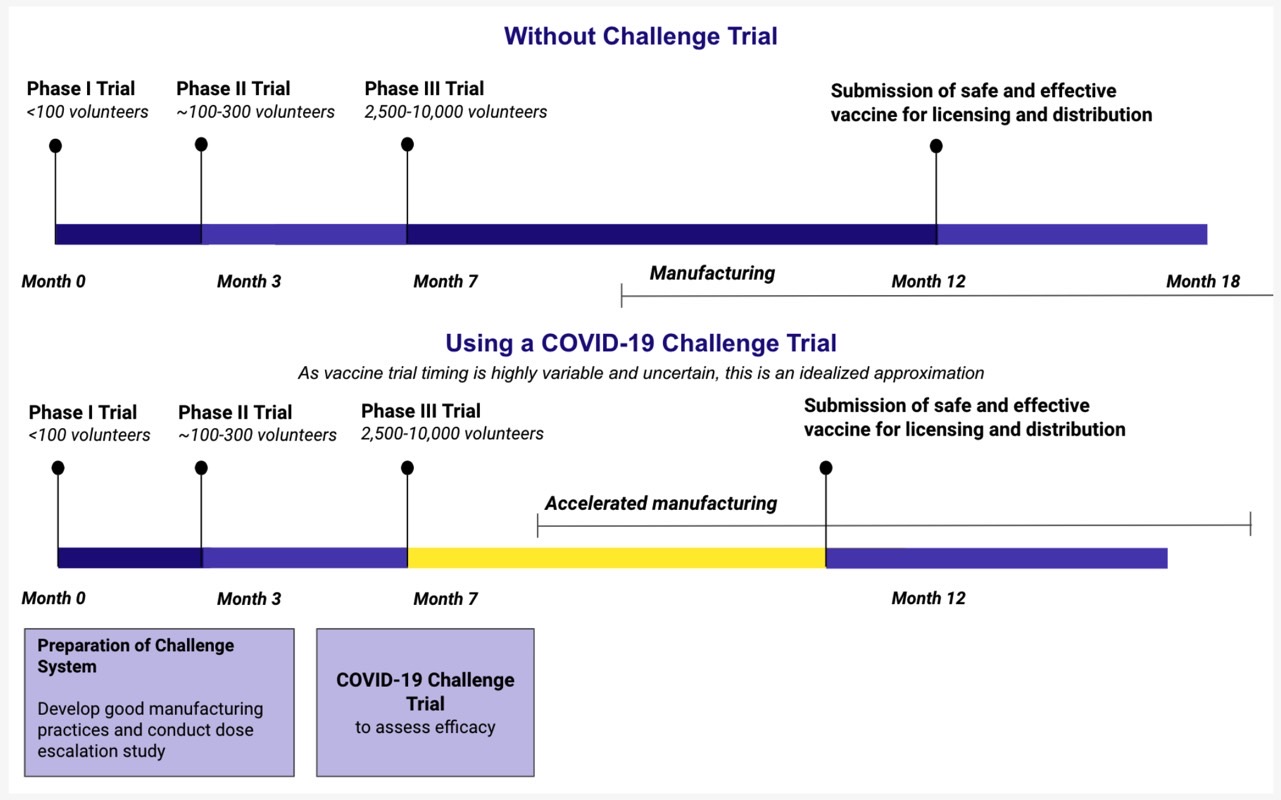
With the number of vaccine candidates increasing at a rapid pace, such challenge trials would not be possible for all proposed drugs. Having to isolate hundreds of people for weeks for each vaccine challenge trial would also rob hospitals of resources that could be used to treat regular COVID-19 patients.
The most recent good news surrounding coronavirus vaccines comes from China, where monkeys were given the virus after the vaccine candidate. The monkeys were not infected or they only developed mild symptoms, depending on the vaccine dose. The same sort of study could be applied to humans if this challenge study idea is approved. But even with that particular drug, which is now in phase I testing, it’s still too early to tell whether it’ll work on humans.
The Hill explains that 35 House lawmakers led by Reps. Bill Foster (D-Ill.) and Donna Shalala (D-Fla.) wrote to the Food and Drug Administration in support of the idea.
“Our situation in this pandemic is analogous to war, in which there is a long tradition of volunteers risking their health and lives on dangerous missions for which they understand the risks and are willing to do so in order to help save the lives of others,” the letter reads.
It’s not just politicians who think the challenge trial idea has merit. Scientists including the vaccinologist Stanley Plotkin who helped invent the rubella vaccine endorse it. Plotkin and New York University bioethicist Arthur Caplan penned an article in Vaccine in support of the idea. Similarly, Harvard University professor Marc Lipsitch co-authored an article in the Journal of Infectious Diseases in support of the idea.
The FDA is resisting this train of thought for now. “The FDA is exploring all possible options to most efficiently advance the development of safe and effective vaccines that will prevent COVID-19,” FDA spokesman Michael Felberbaum told The Hill. “Human challenge studies used to develop a COVID-19 vaccine may present ethical and feasibility issues that can be avoided with the use of animal models.”








