These Illustrations Show How A Coronavirus Vaccine Could Prevent Infection
- As many as 90 vaccine candidates for the novel coronavirus are already in the works, with six of them having hit human trails.
- Not all these vaccine candidates use the same technology to trigger an immune response against COVID-19.
- Nature has illustrated them to explain the differences between the various types of vaccines that could be used for the new disease.
- Visit BGR's homepage for more stories.
Without treatment for COVID-19, all that we can do right now is try to reduce the transmission rate. Social distancing measures, increased hygiene practices, and face masks can all help slow the spread. But unless medication is found to kill the virus in infected patients and prevent life-threatening complications, or a vaccine is developed, we might be in for a long fight with the novel coronavirus.
The good news is that doctors are trialing several drugs that have shown promise and working on an increasing number of vaccines. The vaccines are what would help us get rid of COVID-19 for good, but not all of them work the same way. Thankfully, researchers are studying all sorts of technologies that can kickstart the immune system and train it for a future encounter with the SARS-CoV-2 virus. The following illustrations explain some of them.
Weeks ago, the World Health Organization (WHO) compiled a list of 70 vaccine candidates, of which three had reached the first phase of human trials. The WHO listed all the vaccine makers that embarked on this quest, as well as the various technologies used to develop the vaccines. Nature now says the number of vaccine candidates has reached a new milestone. 90 compounds are in testing, six of which are already being tested on humans, with others having reached the animal testing phase.
Nature has put together a series of images that explain how these vaccines work. The magazine explains that are four main ways to kill the novel coronavirus. Some vaccines will use the inactivated or weakened SARS-CoV-2 strain to generate an immune response. Others will rely on a different weakened virus, like measles or an adenovirus, to transport the SARS-CoV-2 spike protein inside the human body. Then, some drugs rely on DNA or RNA to prepare the immune system for the infection, while other vaccine candidates target certain proteins found on the virus.
The following image shows how virus-based vaccines work. These are products using a version of the virus that can't cause a full-blown infection. Instead, they'll just activate the immune system, which will generate antibodies. We've already talked about one such candidate from China, a Sinovac drug that prevented the COVID-19 infection in monkeys. Seven companies are using this vaccine technology for COVID-19:
Researchers from Oxford came up with their own product that was able to prevent an infection in the same species of primates, but they used a modified version of a different virus, which was weakened beforehand. 25 companies are working on similar viral-vector vaccines.
Another way to generate an antibody response is with the help of a DNA or RNA vaccine. These vaccines will transport the genetic information of the virus inside cells, where they trigger an immune reaction. Such vaccines aren't currently in use for other diseases, but one of the first COVID-19 vaccine candidates to hit human trails comes from Moderna, and it's an mRNA vaccine. Moderna is already preparing for its phase 2 trial that will incorporate even more people. Similarly, one of the vaccines that the Bill and Melinda Gates Foundation is backing up is a DNA drug, made by Inovio. In total, 20 teams are developing DNA or RNA vaccines.
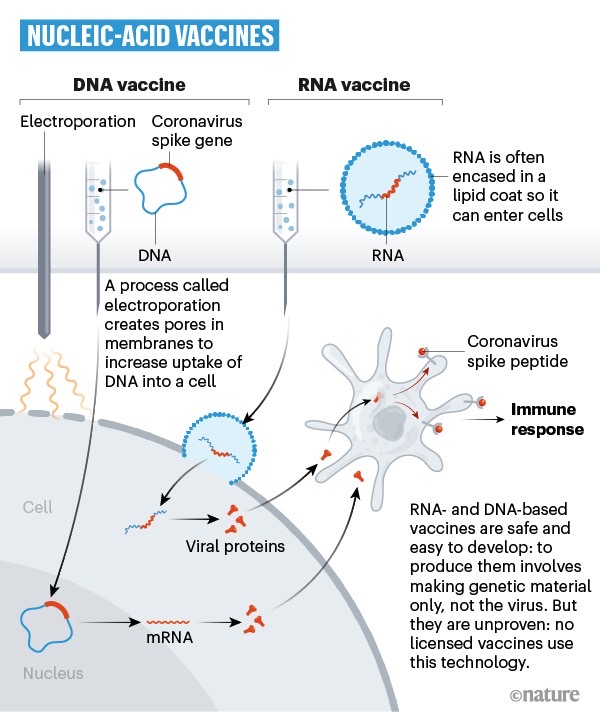
Finally, 28 teams are working on vaccines that would inject the proteins, like SARS-CoV-2's spike protein, into the body to elicit an immune response.
It's unclear which vaccines will work best against the new virus. It's also possible that different vaccine techs prove to be effective against COVID-19. What's reassuring is that SARS-CoV-2 mutations are mild, which means a vaccine would not need to be adapted every year as is the case with the flu. Also promising is the fact that researchers have taken so many different approaches to stopping the virus with a vaccine. We'll have to wait up to 18 months for vaccines to be available to the general public, though the first emergency uses for some of these products could be approved as soon as this fall.
