This Coronavirus Vaccine Might Be Just A Few Months Away From Release
- A new coronavirus vaccine candidate from Germany has entered human trials, and it might be ready for emergency use as soon as this fall.
- Vaccine candidate BNT162 was administered to volunteers in Germany, although healthy American adults will also be included in the trial.
- BioNTech is working with Pfizer on the drug that has the potential of preventing a COVID-19 infection.
- Visit BGR's homepage for more stories.
Remdesivir may be the talk of the town right now because the anti-viral that was initially developed to treat Ebola will become standard care for COVID-19 patients, according to Dr. Anthony Fauci. The drug may not prevent deaths, but the preliminary findings of a massive study show it can reduce the recovery time in patients infected with the novel coronavirus. While COVID-19 complications can still appear, the drug may help patients recover faster and reduce their stays in hospitals. That could be of enormous help to both the patients and the medical centers treating large numbers of COVID-19 patients, especially once a second wave inevitably hits.
The anti-viral isn't a "miracle cure," but those drugs are also in the making. They're vaccines, if you were wondering, and some 90 different candidates are already in the works. A brand new one from Germany just reached the human trials phase, and it could be available to millions of people as soon as this fall.
Before you get too excited, you have to remember that the official line of any government dealing with the COVID-19 outbreak is that a vaccine will not be ready for 12-18 months. At the same time, you'll hear that vaccines may be ready for use this fall or winter, which may sound puzzling. But that's emergency use, or the type of treatment that doesn't apply to the general population. The people fighting the disease in the front lines, including doctors, nurses, police, and other essential personnel, would be the first to get these vaccines. Needless to say, that makes perfect sense. Hopefully, if any vaccine reaches that stage, then it'll mean the vaccine is effective and safe for people, and it's almost ready for mass usage.
German company BioNTech developed a COVID-19 vaccine called BNT162, and the first 12 people were inoculated on April 23rd in Germany. BioNTech partnered with pharmaceutical giant Pfizer to expand the trial to US volunteers, who will get the drug as early as next week. Pfizer might have the medication ready for emergency use this fall.
"The two companies plan to jointly conduct clinical trials for the COVID-19 vaccine candidates initially in Europe and the US, across multiple research sites," Pfizer said in its Q1 2020 earnings report earlier this week. "The companies estimate that there is potential to supply millions of vaccine doses by the end of 2020, subject to technical success of the development program and approval by regulatory authorities, and the potential to rapidly scale up the capacity to produce hundreds of millions of doses in 2021."
BioNTech plans to administer doses ranging from 1µg (microgram) to 100µg to up to 200 healthy volunteers aged 18 to 55, CNN reports. The company isn't just assessing the effectiveness and safety of the drug in this Phase 1/2 study, it's also looking to find the optimal dosage for the drug.
The report doesn't mention what type of vaccine BNT162 is, but a quick look at a WHO listing from a few weeks ago reveals that the only BioNTech/Pfizer vaccine is an mRNA drug that was in a pre-clinical phase as of April 20th. BioNTech and Pfizer's announcements also confirm BNT162 an mRNA vaccine.
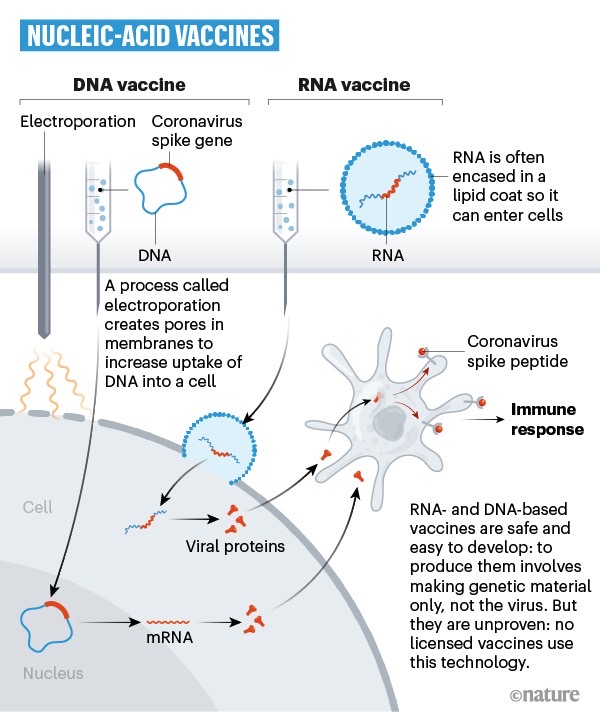
As a reminder, vaccines can employ different technologies to kickstart the immune system and prevent infection from a specific pathogen. Nature earlier this week produced illustrations that show the differences between these vaccines. The image above explains genetic vaccines, including RNA and DNA types.
BioNTech isn't the only company working on an mRNA vaccine that could be ready for emergency use by fall. Moderna has asked permission to start Phase 2 trials for its vaccine candidate, which was the first such drug given to American volunteers more than a month ago. Moderna also estimated that the vaccine will be ready for emergency use this fall, assuming it's effective and safe.
Separately, an Oxford vaccine that uses an attenuated virus to deliver the COVID-19 payload could also be ready this fall.
